|
CHAPTER 2
How Radiation From Atomic Energy Programs
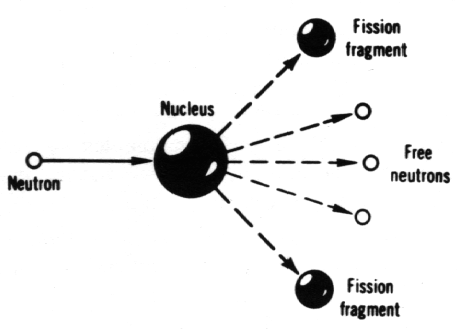
To understand why there is grave concern about nuclear electricity generation, it is necessary to know just how nuclear electric power exposes human beings and other living things to the danger of being irradiated. And, it is essential to understand a few extremely simple points about radioactivity, if we are to bypass the confusion on safety points generated by well-planned propaganda campaigns. The nuclear fission of uranium or plutonium releases enormous energy. This is the energy, ultimately converted to heat, which produces steam to drive turbines and produce electricity. If this were the only energy released when uranium or plutonium atoms split, nuclear electricity might well have been a real boon to mankind, providing electric power for decades or centuries. Unfortunately, the fissioning itself is only the beginning. There is another source of energy involved—after the fissioning is completed—a source which creates the extreme radiation hazard that accompanies nuclear electricity generation. The uranium itself decays or disintegrates very slowly. This is why uranium is still present on earth so long after the earth was formed. Uranium is radioactive, but only feebly so. The kind of uranium (uranium-235) which maintains the chain reaction in a nuclear reactor decays by emitting so-called alpha particles very slowly, so slowly that it takes 710 million years for one-half of the uranium-235 atoms to disintegrate. For any radioactive substance, the time required for one-half of it to disintegrate is called the half-life. Thus, uranium-235 has an extremely long half-life and, consequently, the radioactive hazard of uranium-235 is very small. When a uranium-235 nucleus splits, two (usually) "fission fragments" of the original nucleus are produced. What are these "fission fragments"? They represent what is left after a neutron has disintegrated the uranium-235 nucleus and after some additional neutrons have escaped during the fission process itself. It has been discovered that these fragments are nothing more or less than variant forms of elements commonly occurring in nature. |

|
| A fission reaction. |
|
On the other hand, if this same radionuclide were ingested with radioactively contaminated food or water and distributed uniformly throughout the body tissues, all body organs can be irradiated by it. The case is the same for x-rays or gamma rays. Low-energy x-rays or gamma rays from outside the body may not affect a deeply-situated internal organ. On the other hand, if the radionuclide emitting those same x-rays or gamma rays is taken into the body, it can affect any organ its x-rays or gamma rays reach. (b) Concentration in organs We must recall that the radioactive elements produced in a nuclear reactor behave almost precisely as do their non-radioactive counterparts. For example, radioactive iodine-131 behaves chemically and biologically just as does stable, or non-radioactive, iodine. But chemical elements do differ from each other in how they distribute themselves once they are inside the body. Iodine is interesting because the thyroid gland has a special affinity for iodine. As a result, the thyroid accumulates far, far more iodine from an ingested dose than any other body organ does. The thyroid uses iodine to manufacture its major active hormone, thyroxin. The radioactive forms of iodine (iodine-131 is one) behave just as non-radioactive iodine would when taken in with food, accumulating preferentially in the thyroid gland. As a result, that tissue receives a far higher radiation dosage in rads from the decaying radioactive iodine than other tissues of the body do. Naturally, radioactive iodine has its major biological effects on the thyroid gland, compared with its effect on other cells in the whole body. There is nothing special about the radioactivity of iodine-131 that makes the thyroid gland vulnerable. Precisely the same injurious effect to the thyroid gland could come from x-rays out of an x-ray machine, or from a source of external radioactive cobalt. Only the rads that accumulate in the thyroid gland cells during a particular time matter in assessing possible damage. If half the radiation comes from the outside (as from an x-ray machine) and half from ingested iodine-131 the injury to thyroid cells will be the sum of the rads delivered by both radiation sources. Certain chemical elements taken into the body along with food or water do not concentrate in specific tissues or organs. Instead, they distribute themselves throughout the body. Cesium, produced abundantly in its radioactive form, cesium-137, by uranium fission, does precisely this. It is said to produce whole-body radiation. Again, the radiations from cesium-137, radioactive iodine-131, or any other radionuclide, or x-rays from a machine are comparable. The effect upon cells anywhere in the body depends solely upon the number of rads they absorb in a particular time period. (c) The role of chemical similarity between elements Certain chemical elements are grouped together because they are particularly similar to each other in chemical (and, hence, biological) properties. Lithium, sodium, potassium, rubidium, and cesium represent one such chemical group. Though these elements are by no means identical in chemical or biological behavior, they do show numerous marked similarities in properties, including the way living things use them in their bodies. Potassium is a prominent, vital constituent of the interior of every living human cell. Fish living in fresh water, where the concentration of potassium is very low, may be forced to concentrate the potassium 1000-fold, in order to maintain the concentration necessary to sustain life. Because cesium is chemically quite similar to potassium, the same mechanism also concentrates cesium from such a fresh water source approximately 1000 times. If the cesium in the fresh water happens to be radioactive cesium-137, from a nuclear reactor or other source, then the fish will contain 1000 times more cesium-137 than the fresh water itself, on a weight-for-weight basis. So here is an illustration of how the remarkable similarity in behavior of certain chemical elements can lead to massive concentration of radioactive substances in living tissues, over that existing in the inanimate environment. This can be very serious, not because the particular radioactive nuclide is different in kind from others, but because concentration through such a mechanism finally leads to a high dose in rads to the tissues exposed. Drinking the water might expose one to very little radiation. Eating fish from that water would expose one to 1000 times more cesium-137 radiation, on a weight-for-weight basis. (d) The role of half-life of the radioactive nuclide Some of the radioactive substances that occur as a result of nuclear fission in a nuclear reactor are extremely unstable, half of the radionuclide decaying or disintegrating in a matter of seconds. In contrast, radioactive strontium-90 has a half-life of 25 years, radioactive cesium-137 a half-life of 33 years. But what counts, in terms of biological injury to living beings is the number of rads absorbed in a tissue per unit-time, not the half-life of the particular radioactive nuclide which is irradiating tissue. Thus, 10 rads from a radionuclide having a half-life of 2 seconds is the same as 10 rads from a radionuclide of 30 years half-life. However, there is an important difference between the very short-lived radioactive element and the very long-lived one, in terms of potential harm. For the short-lived nuclide, 10 rads delivered in the course of 5 minutes may be all the radiation that will ever be received by the tissue, simply because essentially all of the radioactive atoms of that radionuclide have decayed away. However, for the long-lived radionuclide, not only can it deliver 10 rads in the course of 5 minutes, but it can continue to deliver this amount, or nearly this amount, of radiation every 5 minutes for years and years. This is the essence of the difference in potential hazard for long-lived versus short-lived radionuclides. Rad for rad, however, they are identical. Another way that half-life of the radionuclide becomes important for biological reasons concerns the chance it has to get out of a nuclear power facility in time to do biological harm. If a radionuclide has a half-life measured in seconds, it is clear that if it can be contained for an hour before being released, essentially all of it is gone before reaching any living tissue. On the other hand, radiostrontium-90 or cesium-137 have half-lives of the order of 25-35 years; not only must we worry about them getting out of a nuclear reactor, but we must worry about them for several centuries! Such radioactive elements must be kept from intersecting with living things, man in particular, at the reactor, during spent fuel transportation and at the fuel reprocessing plant. And finally, the highly radioactive waste must be guarded for something like 500 years. When a radionuclide has a half-life of 30 years, an appreciable amount of radioactivity will persist several hundred years. (e) Whole body radiation versus partial body radiation Obviously, irradiation of the entire body with any number of rads is more serious than irradiation of a particular part, some organ for example. It is not easy to state flatly whether irradiation of one specific part of the body is worse than irradiation of some other specific part. In the case of the male and female reproductive organs, however, the damage done during reproductive years guarantees great harm. Since the gene-containing cells for future generations of humans reside in these organs, irradiation here will cause the genetic (inherited) alterations which can produce mental and physical deformities, and a host of serious diseases in future generations. And since genetic injury is the most serious injury produced by radiation, it is true that irradiation of ovaries and testes is almost as serious as irradiation of the whole body (which, of course, includes the ovaries and testes). For all the remaining organs, in adults at least, cancer is a major hazard and is almost certainly due, in a specific organ, to irradiation received by that organ. At first thought, it might appear that the organs could be ranked by weight to determine the seriousness of the irradiation for cancer induction. The cancer risk for any specified amount of radiation appears more related to the spontaneous cancer risk for that organ than to its size. As we have seen, in most cancer induced by radiation, the radiation directly affects the cells which may become cancerous later. However, from certain animal studies, it appears that radiation in one part of the body can result in a lymph cancer developing elsewhere.[1] This is not common. (f) Age an important factor No factor is of greater importance in considering the implications of delivery of radiation to humans than is age. Direct evidence has been provided by Dr. Alice Stewart of Oxford, England that developing embryos are vastly more sensitive to the cancer and leukemia producing effects of radiation than are adults. In fact, a given amount of radiation increases the risk of future cancer or leukemia 50 times more if delivered to the embryo during gestation than if delivered to adults. Next to the sensitivity of the fetus in utero are children, and then come adults. Unfortunately, even the sensitivity of adults to cancer production by radiation is 10 to 30 times more than "expert" bodies of scientists thought up until the last few years. The embryo presents other special problems too. Radiation, received at a time where the various organs are being formed, can cause a whole organ system to be deformed. For example, early radiation can lead to serious brain injury with resultant mental infirmities. This was seen in Hiroshima. |