back to CNR |
radiation |
rat haus |
Index |
Search |
tree
plain HTML |
ASCII text
formats
Radiation-Inducible Chromosome Injuries:
Some Recent Evidence on Health
Consequences --- Major Consequences
John W. Gofman, M.D., Ph.D., Spring 1992
- Part 1 -- "Permissible" Doses Established in an Ocean of Ignorance
- Part 2 -- "Genetic" versus "Inherited," and Some Other Terms
- Part 3 -- The Pre-Cytogenetic Era, up to 1956
- Part 4 -- The Establishment of Cytogenetics, 1956-1959
- Part 5 -- The Pre-Banding Era of Cytogenetics, 1959-1970
- Part 6 -- The Banding Era of Cytogenetics, 1970-1985
- Part 7 -- The Era of Molecular Cytogenetics, 1985 Onward
- Part 8 -- What Else Will the New Technologies Reveal?
- Reference List

Chromosomes are the structures, in the nuclei of our cells,
which are composed of helical, double-stranded DNA and associated
proteins. The DNA molecules encode our human and individual
genetic heritage. Two types of genetic injury which are readily
caused by ionizing radiation at very low doses and low dose-rates
are chromosomal deletions and translocations.
Recent evidence links a great variety of chromosomal deletions and
translocations with devastating birth defects and mental
handicaps. Nonetheless, pressure to "forgive" more nuclear
pollution --- and thus "forgive" more involuntary exposures to
ionizing radiation --- is reviving in a big way. One consequence
of additional exposure would be additional injury of the
population's chromosomes, our library of genetic information.
1
 "Permissible" Doses Established in an Ocean of Ignorance
"Permissible" Doses Established in an Ocean of Ignorance
The chromosome story is a classic example of how "permissible"
levels of radiation and other pollutants are recklessly established
under the "prove harm" doctrine before technologies even exist for
proving which agents can be the cause of dreadful health effects.
This CNR paper describes the evidence which links chromosomal
deletions and translocations with mental handicap and structural
defects of the heart, kidneys, digestive tract, skeleton, and
genitalia, and it also describes the limits of technology which
have delayed this evidence for so long.
The essay is a non-technical introduction to just a small part of
the story of chromosomal injuries, for it omits any consideration
of health consequences such as cancer, schizophrenia, and metabolic
diseases (for instance, diabetes, hyper-lipidemia, cystic
fibrosis). My next book, in 1994 (Chernobyl Accident:
Radiation Consequences for This and Future Generations (Russian
Language)), will provide detailed evidence and analysis of the
under-estimated health effects which can arise from radiation-induced
chromosome damage. The information also has implications far beyond
nuclear pollution, to the extent that chemicals and viruses (and
possibly other types of radiation) may induce permanent chromosome
injuries too.
The fact that ionizing radiation can break chromosomes has been
"answered" between 1970 and the present day by questioning the
health effects (see the three boxes
[1],
[2],
[3]
in this essay). With respect to this and many other pollutants,
the "prove harm" proponents see nothing wrong about establishing
"permissible" levels of involuntary exposure, despite an ocean of
ignorance regarding the potential, miserable consequences ---
some of which are identified in this essay.
2
 "Genetic" versus "Inherited," and Some Other Terms
"Genetic" versus "Inherited," and Some Other Terms
Among the permanent genetic injuries which can be inflicted by
ionizing radiation are three types:
- Single-gene damage:
Chromosome damage confined to a
segment of DNA representing a single gene.
- Deletions:
Breakage of a chromosome, followed by
permanent loss of part of a chromosome carrying some or many
entire genes, or just part of one gene.
- Translocations:
Breakage of one or more chromosomes,
followed by permanent removal of some or many genes (and partial
genes) from their normal place in the DNA chain; these relocated
DNA segments can end up in an abnormal place within the same DNA
chain or within the DNA of an entirely different chromosome.
All three types of permanent chromosomal injury are now called
"genetic mutations," and types (B) and (C) are also
called "structural chromosome aberrations."
The terms "genetic" and "inherited" are not synonymous. Genetic
injuries or mutations can occur in cell-nuclei
Before conception, in an ancestor's germ cells (sperm or ova).
After conception, during the person's gestation (in-utero).
Anytime during childhood and adulthood.
When genetic mutations occur before conception (inherited) or
during early gestation (not inherited), the health consequences
can be virtually identical. Distinctions are poorly defined
between "genetic diseases," "irregularly inherited disorders,"
"constitutional diseases," "chromosomal disorders," "congenital
diseases," and "birth defects" or "anomalies."
An explosion of new information on these topics has
occurred. Indeed, a large share of all bio-medical research in
recent years has been devoted to the genetic basis of disease
and health, and existing results await coherent assembly and
analysis. Part of the explosion is generated by the Human Genome
Project, in which the U.S. Department of Energy is extremely
active.
3
 The Pre-Cytogenetic Era, up to 1956
The Pre-Cytogenetic Era, up to 1956
The field of chromosome study, broadly, is called
cytogenetics. Although the existence of chromosomes has been
known for over a century, very little progress was made for a long
time.
Chromosomes are not visible, unless you "catch" a cell which is
preparing to divide. Then the very long, string-like chromosomes
"condense" by folding themselves into enormously shorter and
thicker objects. Ordinary stains used in biology showed their
existence, but the objects appeared entangled with each other, and
no one was even able to establish the correct number of human
chromosomes per cell-nucleus during the pre-cytogenetic era.
In 1953, Hsu developed a simple but enormously powerful technical
advance in chromosome studies. When cells are bathed in a
solution with salt-concentration lower than their own
salt-concentration, the cells swell. The chromosomes in cells
preparing to divide become so well separated that quite a few
details of individual, separated chromosomes can be noted, when
the division is halted by a chemical inhibitor and the cells are
flattened on a glass slide. Hsu's advance in laboratory techniques
would soon establish the field of cytogenetics.
The year 1953 was also the year in which Watson and Crick announced
the structure of the gene and DNA helix. The required technologies
for that kind of very sophisticated analysis had become available
before the availability of techniques which would permit us merely
to count the number of structures which carry the genes.
4
 The Establishment of Cytogenetics, 1956-1959
The Establishment of Cytogenetics, 1956-1959
Within three years of Hsu's low-salt cell-preparations, Tjio and
Levan were able to establish conclusively, in 1956, that the
normal number of human chromosomes per cell-nucleus is 46.
The father contributes 23 chromosomes and the mother also
contributes 23 chromosomes to the fertilized ovum, from which
the 46 are replicated in the cell-nuclei of all the descendant
cells --- when everything goes well. There are 22 matched pairs
called autosomes, grouped by letters A-G (for instance, paternal and
maternal B-5 chromosomes). In addition, each cell has a pair of
sex chromosomes which are not necessarily matched (X+X makes the
child female; X+Y makes the child male). Each chromosome has a
region somewhere along its length called the centromere, which
divides the chromosome into a shorter arm (called the p-arm) and
a longer arm (q-arm).
Each pair of undamaged autosomes provides the cell with two
copies of each gene on the autosome --- a full set of this genetic
information from the father and a full set from the mother. In
1956, we were yet to learn that there can be severe consequences
for the children who have either more than two complete
copies or fewer than two complete copies of the genetic
information on both arms of each chromosome. But in 1959, our
ignorance on this matter began to retreat.
5
 The Pre-Banding Era of Cytogenetics, 1959-1970
The Pre-Banding Era of Cytogenetics, 1959-1970
Banding is a technique which will be described in
Part 6. Here we will summarize some insights
which were gained in the pre-banding era.
In Part 2, we described two types of
structural chromosome aberrations (deletions and
translocations). There are also numerical chromosome
aberrations. When an extra copy of one complete chromosome is
present in cells, so that the cells contain 47 instead of 46
countable or "free" chromosomes, the condition is called a
trisomy. When one complete copy of a chromosome is missing,
so that cells have 45 instead of 46 countable chromosomes, the
condition is called a monosomy. Of course, neither condition
could be verified until the normal number of chromosomes was
discovered in 1956.
In 1959, the cause of Down's Syndrome was discovered by
Lejeune and Jacobs to be the presence of a third copy of the
G-21 chromosome in a child's cells. Individuals with Down's
Syndrome almost all suffer from mental handicap and
characteristic facial features, and about 28% also suffer from
a congenital heart defect. Approximately 1 per 700 liveborn
children is a Down's child.
At first, it was assumed that Down's Syndrome required the
trisomy-21 to involve a third copy of the full chromosome in
every cell. We can call this an all-cell, full-chromosome
trisomy-21. It accounts for about 92% of all cases of Down's
Syndrome. The rest of the cases arise from two other types of
trisomy-21. Although we will explain them here, with the
pre-banding era, these other types were not discovered as early
as all-cell, full-chromosome trisomy.
In-Utero Events: Mosaicism
When an infant has 47 chromosomes in every cell, it means that
the numerical aberration was present in the fertilized
ovum. But when only some fraction of a child's cells has
47 chromosomes, it means that the numerical aberration occurred
in a cell which was ancestral to only some of the child's
cells. Thus, the aberration occurred during gestation,
in-utero. Individuals with two types of cells (some with 46
chromosomes, some with 47) are mosaics, and they have a some-cell,
full-chromosome trisomy. For Down's Syndrome, mosaicism accounts
for about 2.7% of the cases.
Translocations and Partial Trisomies
It has been shown that Down's Syndrome can be caused also by a
structural chromosome aberration --- one which is readily
induced by ionizing radiation. We mean the translocation (see
Part 2). An estimated 5.8% of all cases are
due to a translocation in one ancestor of a child. Although a
thorough explanation would require more space than we have here,
Figures 1 and 2 may convey a sense of
the problem.
Figure 1 depicts a normal E-18 chromosome and a normal B-5
chromosome. Figure 2 depicts their possible status after a
translocation. When a chromosome carries a mixture of
information belonging to more than one chromosome, its name is
set by the information around its centromere (depicted by the
black area). In Figure 2, the chromosome on the left is called
the E-18, and B-5 is on the right.
The condition of partial trisomy arises for a child as
follows.
Suppose that both the mother and father transmit normal E- 18
chromosomes to their child. But suppose that in the
transmission of B-5 chromosomes, one parent transmits the
damaged B-5 chromosome from Figure 2. It carries translocated
genes belonging to the q-arm of the E-18 chromosome. The child,
whose numerical count of separate chromosomes is the normal 46,
will nonetheless have three copies of part of the genetic
information on the q-arm of the E-18 chromosome. The child will
have a partial trisomy-18. This structural aberration can be
described as an all-cell 18q trisomy.
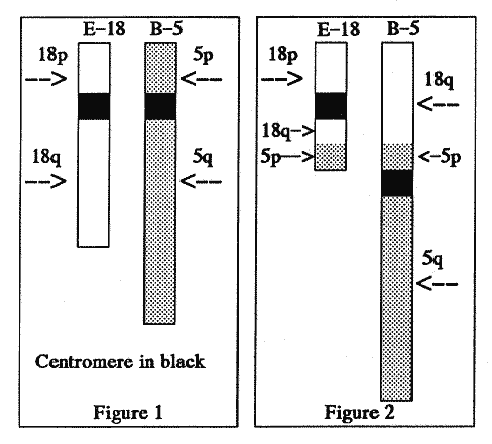
Translocations and Partial Monosomies
In the example above, the child simultaneously has a partial
monosomy because the child has received a B-5 chromosome which
lacks part of its p-arm and lacks the genetic information which
was on it. Even though the other parent sends the child a normal
B-5 with a complete p-arm, the child will have only one
copy (instead of the normal two copies) of some genetic
information belonging to the p-arm. This condition can be
described as an all-cell 5p monosomy. Because the
chromosome-count will be the normal 46 per cell, this is not a
numerical aberration.
Emphasis belongs on the fact that the effect of a partial
monosomy is no different from an inherited deletion (see
Part 2). The net effect is an all-cell
deficit of chromosomal information. The deficit may or may not
be limited to genes which code for specific enzymes. The deficit
will often include (A) some segments of DNA which have
presently unknown but presumably important functions, and
(B) some chromosomal proteins whose functions are
presumably important too.
The Discovery of Trisomies Additional to Down's Syndrome
In 1960, Patau presented the first clinical observation of a
full-chromosome trisomy-13 patient. Since then, the frequency
has been estimated between 1 case per 4,000 and 1 case per 10,000
live births. The clinical features of trisomy-13 include
(percentage of cases): Mental handicap 100%, undescended
testicles 89% of the males; abnormally small jaw 87%; eye
defects 88%; low-set or malformed ears 85%; heart defects
79%; apparent deafness 77%; cleft palate 77%; extra fingers
77%; kidney defects 66%; seizures 41%.
And also in 1960, Edwards described the first patient with a
full-chromosome trisomy-18. The frequency is now estimated at 1
case per 8,000 live births. The clinical features of trisomy-18
include (percentage of cases): Mental handicap 98%; undescended
testicles in 77% of males; abnormally small jaw 95%; low-set or
malformed ears 95%; congenital heart defects 95%; kidney defects
60%; prominent heel-bone 74%; elongated (front to back) skull 87%.
|
Box #1, A Contrast in Warnings: Examples from 1969-1970
 -- On October 29, 1969, at the IEEE Symposium on Nuclear Science,
Gofman and Tamplin upset the nuclear community by
their call for an immediate 90% reduction in the radiation guidelines set
in 1960 by the Federal Radiation Council (FRC) for members of
the public. These were "permissible" doses of 0.17 to 0.5 rem
each year. A few months later, Gofman and Tamplin
challenged the government's authority to legalize involuntary radiation
doses at any level.
-- On October 29, 1969, at the IEEE Symposium on Nuclear Science,
Gofman and Tamplin upset the nuclear community by
their call for an immediate 90% reduction in the radiation guidelines set
in 1960 by the Federal Radiation Council (FRC) for members of
the public. These were "permissible" doses of 0.17 to 0.5 rem
each year. A few months later, Gofman and Tamplin
challenged the government's authority to legalize involuntary radiation
doses at any level.
 -- In testimony presented to Congress in June 1970,
Gofman and Tamplin posed a series of detailed questions for the FRC
chairman concerning genetic injuries from the permissible dose.
-- In testimony presented to Congress in June 1970,
Gofman and Tamplin posed a series of detailed questions for the FRC
chairman concerning genetic injuries from the permissible dose.
"We are just beginning to learn the meaning of a variety
of chromosomal anomalies, including deletions and translocations
for numerous aspects of human health and disease . . . Does it
make you feel at all uneasy that this spectacular field of human
cytogenetics is now in its infancy, after all the
decisions had been made which led to the setting of FRC
guidelines for radiation exposure of
populations?" (Gofman and Tamplin 1970, p.1554).
 -- On August 5, 1970, Dr. John Totter, director of the
Bio-Medical Division of the Atomic Energy Commission (AEC),
was asked about radiation-induced chromosome injury during
his testimony before a Senate hearing chaired by Sen. Mike
Gravel.
-- On August 5, 1970, Dr. John Totter, director of the
Bio-Medical Division of the Atomic Energy Commission (AEC),
was asked about radiation-induced chromosome injury during
his testimony before a Senate hearing chaired by Sen. Mike
Gravel.
Sen. Gravel: "What happens to cells [if they receive 10 rads]?"
Dr. Totter: "You would see chromosome breakage."
Sen. Gravel: "Would that be significant in your terms?"
Dr. Totter: "Well, it is undoubtedly significant, but what
significance it has, we don't know" (Totter, p.677).
Nonetheless, the AEC vigorously opposed any reduction in the
permissible dose (AEC Oct. 31, 1969, pp.203-209 --- an example of
many subsequent statements). Unofficially, there was talk of
increasing the permissible dose. Back then, the AEC had
ambitious plans to build "a plutonium economy," to license 800 to
1000 large nuclear power plants by the year 2000 (AEC 1970,
p.685), and to loosen natural gas in the Rocky Mountains by
exploding hundreds of underground nuclear bombs.
The plans and the public's exposure to the permissible dose would
probably have become a reality, if it were not for rising public
concern in the 1970s over the potential health effects.
|
Later, with the advance of technology, it was discovered that
both trisomy-13 and trisomy-18 --- like trisomy-21 --- also can
occur as mosaics (in-utero) and as partial trisomies (inherited
as a result of translocations).
The Linking of Cause with Consequence
No one is claiming that all individuals who have the health
effects listed above are cases of trisomy-13, -18, or -21. Many
additional genetic causes of these health problems have been
discovered, and it is possible that some cases arise without any
genetic injury at all. Then how can anyone be sure that a
trisomy causes the problems of trisomic individuals?
Whenever a variety of causes might produce the same health
effect, two types of study can establish causation.
In a prospective cohort study, you start with a suspected
cause and then you measure the occurrence of presumed
consequences. You measure the health of one group which has
trisomic cells (a presumed cause of the listed health effects)
and another group which does not have trisomic cells,
and you discover which group has the higher rate of the health
effects. The frequency of mental handicap, for example,
approaches 100% in the trisomic individuals, while the
frequency is certainly lower in the general population.
In a retrospective case-control study, you start with presumed
health consequences and then you measure the occurrence of a
suspected cause. You measure the rate of trisomic cells in one
group which has the health effects (a presumed consequence of
trisomy) and in another group which does not have these
health effects, and you find out which group has the greater
frequency of trisomic cells (a presumed cause). There is no
doubt that the frequency of 47 chromosomes is higher among the
persons who have the health effects listed above. Indeed, the
rate of full-chromosome trisomy among persons who lack
such health effects is so low that we are unaware of a single
known case.
The First Discoveries of Deletion Syndromes
All-cell, full-chromosome trisomies were open to study in the
early era, since this was a matter of just counting
chromosomes. But the opposite possibility --- namely, a
deficit of certain chromosomal information --- was not
nearly so easily studied, since only arm-lengths and
centromere positions were available to identify such losses.
Cri-Du-Chat Syndrome.
Nonetheless, by 1963 progress was
underway when Lejeune and co-workers described the first three
cases of the 5p partial monosomy or deletion syndrome. It was
called Cri du Chat Syndrome because infants with it have a
peculiar, cat-like mewing cry. Hundreds of cases were
subsequently reported, and they are missing 30% to 85% of the
short arm of the B-5 chromosome. The disorder is
severe. Besides the cry, clinical features in most cases
include profound mental handicap, small head, low-set ears, and
growth failure.
|
Box #2, A Contrast in Warnings: Examples from 1980-1981
 -- The BEIR Committee replaced the Federal Radiation Council. In
1980, what sort of warning did it issue concerning
radiation-induced chromosome damage? First, it grouped "small
deletions" with single-gene mutations, and then
stated: "Disorders due to chromosomal aberrations . . . will
amount to fewer than 10 anomalies per million liveborn, and
most subcommittee members felt that the true value may be near
zero. (BEIR-3, Chapter 4 Summary). Instead
of any warning flag, a strong suggestion of no effect was produced.
-- The BEIR Committee replaced the Federal Radiation Council. In
1980, what sort of warning did it issue concerning
radiation-induced chromosome damage? First, it grouped "small
deletions" with single-gene mutations, and then
stated: "Disorders due to chromosomal aberrations . . . will
amount to fewer than 10 anomalies per million liveborn, and
most subcommittee members felt that the true value may be near
zero. (BEIR-3, Chapter 4 Summary). Instead
of any warning flag, a strong suggestion of no effect was produced.
 -- In 1981, the book Radiation and
Human Health (Gofman 1981) bristled with warnings
in its last 150 pages of analysis. For instance:
-- In 1981, the book Radiation and
Human Health (Gofman 1981) bristled with warnings
in its last 150 pages of analysis. For instance:
- "The author's opinion is that small deletions occurring in-utero
produce mosaicism which will prove to be an important basis for
congenital anomalies. This is an opinion, not a fact. It is a
fact that the technology for studying this question is not
currently available" (p.721-22).
- "With respect to both deletions and translocations, the author
would like to warn the reader about a very poor practice in a
great deal of the medical and scientific literature. The
technology for recognizing and measuring relatively small
deletions and translocations is most appropriately described as
primitive, even after taking the new banding techniques into
account. The large majority of small deletions and
translocations can not possibly be recognized with the use of
available technology. Yet many authorities treat these
injuries as though they simply do not occur" (p.766).
- "The extent to which the genetic-chromosomal effects of
radiation are under-estimated, just on the basis of a total
absence of appreciation of the deletion problem, can not be
known. Any guesstimate would be highly speculative. But the
author of this book would not be at all surprised if future
evidence showed that the `deletion cost' from radiation exceeds
most of the costs which have been estimated already" (p.843).
|
Wolf-Hirschhorn Syndrome.
In 1965, the 4p partial monosomy or
deletion syndrome was discovered by Wolf. Between 10% to 80% of
the short arm of chromosome B-4 is missing. Clinical features of
this Wolf-Hirschhorn Syndrome include severe mental handicap,
seizures, delayed psychomotor development, pre-natal and
post-natal growth failure, and multiple malformations such as
cleft palate, cleft lip, congenital heart malformations, genital
abnormalities in males, defects of the urinary tract, skeletal
abnormalities of fingers, hips, spine. As we shall see in
Part 7, special facial features are
associated with Wolf-Hirschhorn Syndrome, too. About 40% of
infants who are born with the 4p deletion syndrome die in infancy
or childhood.
6
 The Banding Era of Cytogenetics, 1970-1985
The Banding Era of Cytogenetics, 1970-1985
The banding era began around 1970, when special stains made it
possible to start differentiating segments (or bands) along
chromosomal arms. Gradually it became possible to distinguish
about 400 pairs of bands (total) among the 46
chromosomes. Advanced, high-resolution banding techniques today
extend the total to over 800 pairs. The naming of each band
begins with the number of the chromosome, then the arm (p or q),
and then single digits indicating large regions. Number 1 is
always the region closest to the centromere, and then additional
digits indicate sub-sections of specific regions.
Banding made it possible to start finding and correctly
identifying translocations and deletions. In the pre-banding
era, the true identity of the injured chromosomes was easily
mistaken, because they acquired new shapes and sizes.
Thanks to the development and improvement of banding techniques,
de Grouchy and Turleau were able to publish a second edition of
their remarkable Clinical Atlas of Human
Chromosomes in 1984. Based on the worldwide literature
of reported cases, the Atlas demonstrates the discovery of
partial trisomies or partial monosomies (deletion syndromes)
involving every autosome. There is not enough space
here even to list them all --- over 70 types known by 1984.
Are these structural chromosomal aberrations associated with
important health effects? Mental handicap at various levels is
one feature shared by almost all 70 types. A few examples follow:
- For chromosome 3, for instance, there is a partial trisomy
involving the 3q2 region. It is associated with severe mental
handicap, heart defects in about 33% of cases, abnormalities of
kidneys and digestive tract "frequently," skeletal abnormalities
at "many sites," and genital abnormalities in males "always" and
in females "most cases."
- For chromosome 3 also, there is a partial trisomy of the
p-arm from 3p2 to the distal end. This is associated with severe
mental handicap, heart defects in about 75% of reported cases,
skeletal abnormalities at many sites, and genital abnormalities
in all males.
- On chromosome 3 also, there is a partial monosomy
involving the 3p2 region. This is associated with very severe
mental handicap skeletal abnormalities at several sites, and
genital abnormalities in both sexes.
The Issue of Causation
This type of evidence in the Atlas, accompanied by some photos
of the infants, is virtually screaming at the
world: CAUTION ! Structural
chromosome aberrations ---
readily inducible by ionizing radiation --- can cause extremely
serious mental handicap and other birth defects. And yet, in
some circles, denial or "we don't know the meaning" is still
heard (Box #3).
Thus, we expect a challenge from some circles to the presumption
that the chromosome aberrations are causing the handicaps
described in the Atlas --- a presumption which is made in the
Atlas and elsewhere, and a presumption which I predict will be
systematically validated in the future.
Consider that there is a continuum of genetic mutations. At one
end, we learned that an all-cell full-chromosome trisomy
causes serious handicaps. At the other end, we know that
a single-gene mutation can cause devastating health
effects, such as cystic fibrosis and Huntington's Disease. Every
few weeks now, the genetic basis of an additional disease is
announced. Would it make sense for anyone to deny that partial
trisomies and partial monosomies, which lie in the realm between
single-gene mutations and full trisomies, have a causal
relationship with the associated health effects described in the
Atlas?
7
 The Era of Molecular Cytogenetics, 1985 Onward
The Era of Molecular Cytogenetics, 1985 Onward
Very strong evidence in favor of causality is provided by a
medical mystery whose solution was described during
1991 by Michael Altherr and co-workers
in the American Journal of Human Genetics. The
solution depended not only on human
tenacity, but also on the availability of the new laboratory
technologies in molecular biology such as RFLP (Restriction
Fragment Length Polymorphism) and FISH (Fluorescence In-Situ
Hybridization) --- which we will not attempt to describe in
this paper.
The mystery involved a female child with the facial features
of the Wolf-Hirschhorn Deletion Syndrome noted at
birth. Serious additional abnormalities included a septal
defect between the cardiac auricles. On the basis of the
facial features, the diagnosis of Wolf-Hirschhorn was
considered, but an ordinary chromosome analysis detected no
abnormality of chromosome 4.
At age one, the child had heart surgery, and as she grew
older, her facial features increasingly had the
characteristics of Wolf-Hirschhorn Syndrome. So a
high-resolution chromosome analysis was performed on her and
on both parents. But even the best banding technologies in
cytogenetics could not provide a conclusive answer
(see Box #2).
Altherr persevered. First he tried RFLP, with DNA probes for
seven different segments within the most distal band of
chromosome 4's p-arm. These DNA probes were able to
establish that the child did not inherit a maternal copy for
two of the segments. When Altherr also used FISH on the
mother's chromosomes, he discovered that she had a small part
of 4p translocated onto the p-arm of chromosome 19. When she
transmitted only 23 chromosomes to her daughter, the daughter
received the copy of B-4 which was missing some information,
but not the copy of chromosome 19 which carried the missing
information --- and which spared the mother from the obvious
health effects.
Which is the more reasonable conclusion from this
story: (A) the very small 4p deletion in this child
caused the characteristic abnormalities observed in
other cases of Wolf-Hirschhorn 4p Deletion Syndrome, or
(B) the very small 4p deletion was present in this
particular child just by coincidence?
|
Box #3. A Contrast in Warnings: Examples from 1988 to Now
 -- Depreciation of chromosome aberrations occurs in
Dr. Thomas Luckey's 1991 book, the
thesis of which is that good health requires more
radiation exposure, not less. Going even further
than Dr. Totter (Box #1), Dr. Luckey
says, "Although chromosomal aberrations are proportional
to radiation dose and appear after very low doses of
radiation, no medical diseases are associated with
these changes" (p.77).
-- Depreciation of chromosome aberrations occurs in
Dr. Thomas Luckey's 1991 book, the
thesis of which is that good health requires more
radiation exposure, not less. Going even further
than Dr. Totter (Box #1), Dr. Luckey
says, "Although chromosomal aberrations are proportional
to radiation dose and appear after very low doses of
radiation, no medical diseases are associated with
these changes" (p.77).
 -- In 1990, the BEIR-5 Committee made some major moves
toward realism with respect to admitting high spontaneous rates
of genetically-related diseases and afflictions. On the other
hand, when it came to assigning responsibility to radiation, the
BEIR-5 Report sent mixed messages. For instance:
-- In 1990, the BEIR-5 Committee made some major moves
toward realism with respect to admitting high spontaneous rates
of genetically-related diseases and afflictions. On the other
hand, when it came to assigning responsibility to radiation, the
BEIR-5 Report sent mixed messages. For instance:
"Although chromosome aberrations can be induced by relatively
low doses of radiation . . . the health implications, if any,
of an increase in the frequency of such aberrations in
circulating lymphocytes is uncertain" (p.34).
Uncertain? If aberrations increase in a population's
lymphocytes because of whole-body irradiation (from either
natural or man-made sources), the aberrations also increase in
all other cells, including the germ cells. "Health
implications" are described in the text.
 -- Analysts at RERF write extensively about the
Hiroshima-Nagasaki children who were in-utero during the
bombings and who showed an elevated frequency of mental
handicaps. RERF is the foundation which controls the A-Bomb
Study for the U.S. Dept. of Energy and the Japanese Ministry
of Health. In five papers published in 1988
through 1991, the RERF analysts speculate for pages about how
radiation could have caused the mental handicaps --- without
even mentioning radiation-induction of chromosome injuries.
-- Analysts at RERF write extensively about the
Hiroshima-Nagasaki children who were in-utero during the
bombings and who showed an elevated frequency of mental
handicaps. RERF is the foundation which controls the A-Bomb
Study for the U.S. Dept. of Energy and the Japanese Ministry
of Health. In five papers published in 1988
through 1991, the RERF analysts speculate for pages about how
radiation could have caused the mental handicaps --- without
even mentioning radiation-induction of chromosome injuries.
 -- The contrast is stunning between the depreciation of
chromosome injuries in some circles, versus the evidence
described in this 1992 essay.
-- The contrast is stunning between the depreciation of
chromosome injuries in some circles, versus the evidence
described in this 1992 essay.
|
Altherr and co-workers comment (1991,
p.1235), "This provides the first evidence, in chromosome 4p,
of a molecular deletion due to a subtle, inherited translocation
leading to the Wolf-Hirschhorn phenotype. Such subtle
translocations may become an important mechanism for some
recurrent genetic defects." We could hardly have conjured up a
better-matching and independent agreement with our own warnings
and predictions of 1970 and 1981.
8
 What Else Will the New Technologies Reveal?
What Else Will the New Technologies Reveal?
The Altherr report confirms a logic which may be self-evident
to many objective analysts in this field: If single-gene
mutations can cause drastic health consequences, then surely
small, sub-visible deletions (either inherited or occurring
early in gestation) can also cause them. In my opinion, if
any radiation expert today were to say "We do not know the
significance of small deletions and translocations," it would
be a strangeness lasting 20 years too long.
Readers may consider the estimate of Dallapiccola and Forabosco
(1987, p.25): "A chromosome contains about 100 million
base-pairs of DNA. Any visible deletion of a chromosome
involves at least 2% to 5% of a chromosome. A deletion would
involve enough space for 2 million base pairs, or about 50
genes (a gene typically takes up about 40,000 base pairs of
space)." The point is that chromosome injuries can delete
multiple genes and still be completely undetectable by
the best banding technologies.
If readers consider the limitless variety of micro-deletions
and translocations which may exist --- undetected --- in
today's population, then they may agree with our prediction:
The new discoveries being made with molecular biological
techniques will confirm that a large part of the congenital
defects of unknown origin, and a large part of the irregularly
inherited diseases of unknown origin, are really consequences
of deletions and translocations at the sub-microscopic
level. The Altherr report is only the very tip of an iceberg
which will be seen more fully in the next decade.
This "iceberg" should be taken into account today when
people discuss "permissible" levels of involuntary exposures to
possible chromosome-breakers and to proven
chromosome-breakers such as ionizing radiation.
Reference List
AEC 1969 (October 31).
James T. Ramey and Dr. Theos J. Thompson, both commissioners of the
U.S. Atomic Energy Commission, in Environmental Effects of Producing
Electric Power, Hearings Part 1: October 31, 1969 before the Joint
Committee on Atomic Energy, 91st Congress, first session,
pp.203-209. 1969. Their testimony opposing any reduction in the
permissible dose is also quoted in Gofman + Tamplin
1971, pp.133-140.
AEC 1970 (August 5).
Clarence E. Larson and James T. Ramey, both commissioners of the
U.S. Atomic Energy Commission, in Underground Uses of Nuclear
Energy, Hearings Part 2 on Bill S.3042: August 5, 1970 before
the Subcommittee on Air and Water Pollution of the Committee on
Public Works, U.S. Senate, 91st Congress, second session, p.685. 1970.
Altherr 1991.
Michael R. Altherr + 7 co-workers, "Molecular Confirmation of
Wolf-Hirschhorn Syndrome with a Subtle Translocation of Chromosome
4," American Journal of Human Genetics Vol.49: 1235-1242. 1991.
BEIR 1980, or BEIR-3.
The BEIR-3 Report, sponsored by the U.S. Government and authored by
the Committee on the Biological Effects of ionizing Radiations (Edward
P. Radford, Chairman). The Effects on Populations of Exposure to Low
Levels of Ionizing Radiation. (National Academy Press, 2101
Constitution Avenue, NW, Washington DC 20418, USA.) No index. 1980.
BEIR 1990, or BEIR-5.
The BEIR-5 Report, sponsored by the U.S. Government and authored by
the Committee on the Biological Effects of ionizing Radiations
(Arthur C. Upton, Chairman). Health Effects of Exposure to Low
Levels of Ionizing Radiation. 421 pages. ISBN 0-309-03995-9. (National
Academy Press, 2101 Constitution Avenue, NW, Washington, DC 20418, USA.)
Dallapiccola 1987.
B. Dallapiccola + A. Forabosco, "Human Micro-Cytogenetics," Acta Medica
Auxologica Vol. l9: 5-33. 1987.
De Grouchy 1984.
Jean de Grouchy (M.D.) + Catherine Turleau (M.D.), Clinical Atlas of
Human Chromosomes, Second Edition. 487 pages. A Wiley Medical
Publication. (John Wiley and Sons, New York City NY, USA.) 1984.
Gofman + Tamplin 1969 (October 29).
John W. Gofman + Arthur R. Tamplin, "Low Dose Radiation and Cancer,"
paper presented at the IEEE Nuclear Science Symposium, San
Francisco. In IEEE Transactions on Nuclear Science, Vol. NS-17
Number 1, Feb. 1970: 1-9. (Institute of Electrical and Electronics
Engineering, New York City NY, USA.)
Gofman + Tamplin 1970 (April 22).
John W. Gofman + Arthur R. Tamplin, "Can We Survive the Peaceful Atom?",
paper GT-120 presented at the Environmental Teach-In of the First "Earth
Day," University of Minnesota, Minneapolis. Reprinted in Earth Day ---
The Beginning; A Guide For Survival, compiled and edited by the staff
of Environmental Action, Washington, DC. Bantam Book number
553-05822-125. Arno Press, Inc., New York City, USA. Also available in
Underground Uses of Nuclear Energy, Hearings Part 2 on Bill
S.3042: August 5, 1970 before the Subcommittee on Air and Water Pollution
of the Committee on Public Works, U.S. Senate, 91st Congress, second session,
pp.1509-1522. 1970.
Gofman + Tamplin 1970 (June 29).
John W. Gofman + Arthur R. Tamplin, "Questions for Dr. Paul Tompkins,
Director of the Federal Radiation Council," in Underground Uses of
Nuclear Energy; the full reference is provided above in the
April 1970 entry; pp.1538-1559. Warnings
about the consequences of chromosome damage are also given in
Gofman + Tamplin 1971 (especially
Chapter 3).
Gofman + Tamplin 1971. Re-issued in 1979.
Gofman 1981.
John W. Gofman, Radiation And Human Health. 908 pages. ISBN
0-87156-275-8. (Sierra Club Books, 100 Bush Street, 13th floor,
San Francisco, CA 94104, USA.) 1981.
Luckey 1991.
Thomas D. Luckey, Radiation Hormesis. 306 pages. ISBN
0-8493-6159-1. (CRC Press, 2000 Corporate Blvd. NW, Boca Raton FL
33431, USA). 1991.
Otake 1988-a (May).
Masanori Otake + Hiroshi Yoshimaru + William J. Schull, Severe Mental
Retardation Among The Prenatally Exposed Survivors Of The Atomic Bombing
Of Hiroshima And Nagasaki: A Comparison Of The T65DR And DS86 Dosimetry
Systems. RERF Technical Report TR-16-87, printed May 1988. (Radiation
Effects Research Foundation. Hiroshima, Japan.) 1988.
Otake 1988-b (August).
Masanori Otake + William J. Schull + Yasunori Fujikoshi + Hiroshi
Yoshimaru, Effect On School Performance Of Prenatal Exposure To Ionizing
Radiation In Hiroshima: A Comparison Of The T65DR And DS86 Dosimetry
Systems. RERF Technical Report TR-2-88. (Radiation Effects Research
Foundation, Hiroshima, Japan.)
Otake 1991.
Masanori Otake + William J. Schull + Hiroshi Yoshimaru, "Brain Damage
among the Prenatally Exposed," Journal of Radiation Research
(TOKYO), Supplement 32: 249-264. 1991.
RERF analysts: Five papers on mental retardation.
Schull 1990.
William J. Schull + Stata Norton + Ronald P. Jensh, "Ionizing Radiation
and the Developing Brain," Neurotoxicology And Teratology
Vol.12: 249-260. 1990.
Totter 1970 (August 5).
John Totter, director of the Bio-Medical Division of the U.S. Atomic
Energy Commission, in Underground Uses of Nuclear Energy Hearings
Part 2 on Bill S.3042, August 5, 1970 before the Subcommittee on Air and
Water Pollution of the Committee on Public Works. U.S. Senate. 91st
Congress, second session, p.677. 1970.
Yamazaki 1990 (August 1).
James N. Yamazaki + William J. Schull, "Perinatal Loss and Neurological
Abnormalities among Children of the Atomic Bomb: Hiroshima and Nagasaki
Revisited 1949-1989," Journal Of The American Medical Assn. Vol.264,
No.5: 605-609.
|
AUTHOR: John
W. Gofman is chairman of
CNR; professor emeritus of Molecular and Cell Biology at the
University of California, Berkeley; founder in 1963 of the
Bio-Medical Research Division of the Livermore National
Laboratory; author of four scholarly books on health effects
from ionizing radiation (1981, 1985, 1990, and 1994 in progress).
|
# # # # #
This document is also available in
plain
HTML and
ASCII text
formats
and can always be found at http://www.ratical.org/radiation/CNR/RICI.html
back to CNR |
radiation |
rat haus |
Index |
Search |
tree


